Medical Device Product Development
At a Glance
Innovation with the patient at heart
Prodigy’s industry-leading best practices are driving patient outcomes across the medical field. We are experts in understanding your unique needs – whether it be end-to-end product development management and execution or more niche expertise to drive your existing projects to the next level. Prodigy is perfectly positioned to bring our cross-vertical expertise to the medical field. Best practices from across the variety of industries we serve are put to work to benefit your medical projects and give you an uncompromising edge over the competition. We are first and foremost problem solvers. Our project management expertise has proved invaluable to our customers – 97% of whom return to us time and again to deliver the speed and expertise they need to maintain leadership.
We bring a wealth of experience and a deep understanding of the medical device design process to each project. Our approach combines innovation with risk mitigation, ensuring that our designs meet industry standards and reach the market swiftly and safely. We are dedicated to crafting medical devices that push the boundaries of technology while prioritizing patient safety and regulatory compliance.
Clinical Applications
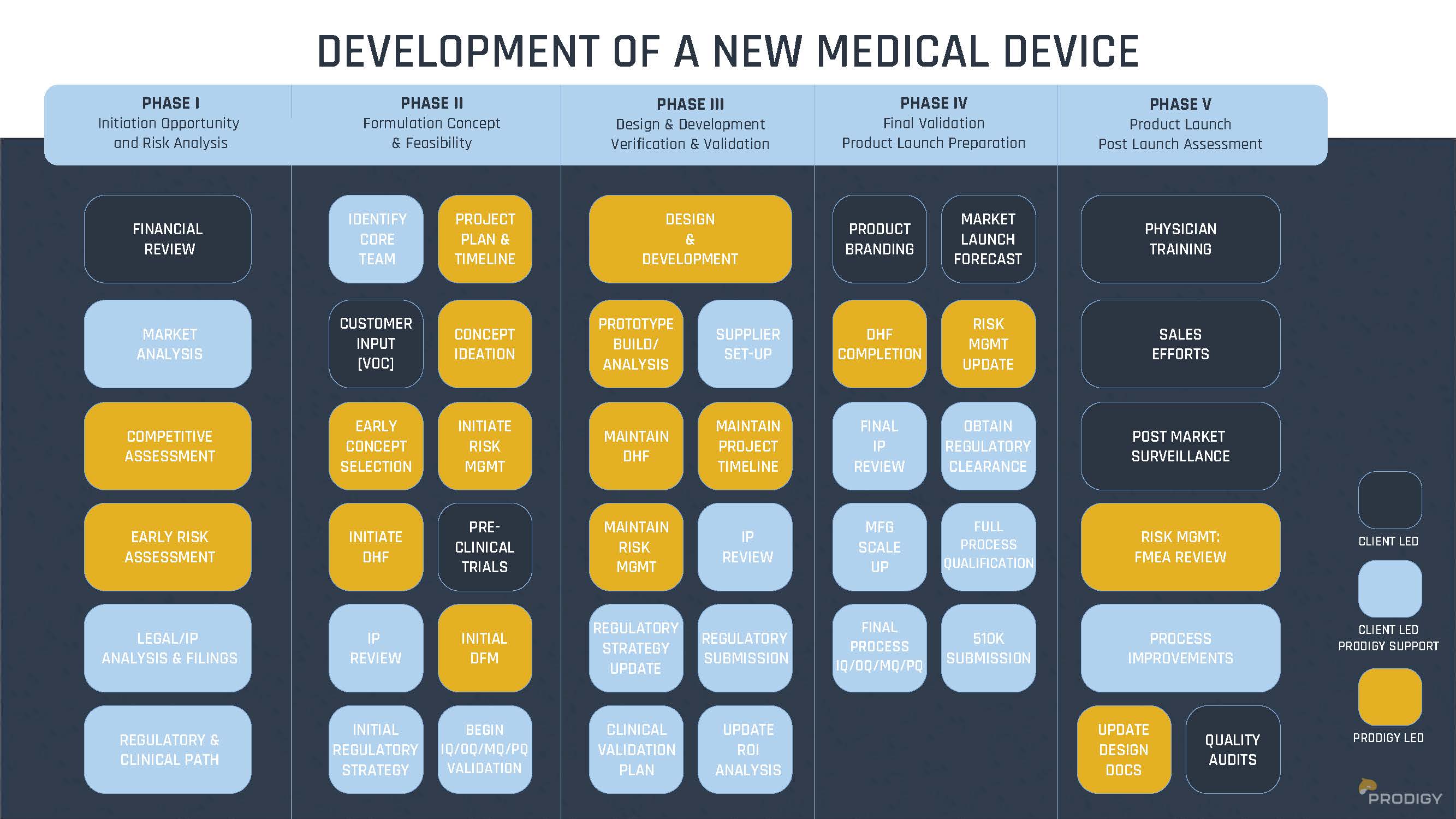
Our Process
At Prodigy, our product development process is designed to enhance value, drive efficiency, and help you maintain a competitive edge. Whether refining an existing product or launching something entirely new, we provide expertise at every stage of development. We adapt our approach to meet your needs—whether you require full end-to-end development or assistance at a specific phase.
- Business Vision
- Brainstorming & Ideation
- Design & Development
- Analysis
- Prototyping
- Testing
- Design Release & Manufacturing Support
Medical Innovation Group

Eugene Chung, Chief Medical Officer
Dr. Chung is an invasive cardiologist and a nationally recognized heart failure specialist. Former Heart and Vascular Service Line Director and Chief of Cardiology at The Christ Hospital, he currently serves as Director of Outreach and Innovation. Dr. Chung is also the Chief Medical Officer for Prodigy. In this role, Dr. Chung guides clinical strategy across Prodigy’s MedTech portfolio, ensuring that device development is clinically informed, regulatory-ready, and aligned with unmet needs in patient care. He also collaborates closely with engineering and product teams to accelerate the translation of clinical insights into impactful medical technologies.
-1-1-1.jpeg)
Geoff Willis - Principal Engineer, Medical Innovation
Geoff Willis is a Principal Mechanical Design Engineer with over 30 years of experience in product development of medical devices and consumer products. He has strong expertise in product design and advanced R&D management. Geoff received a Bachelor of Science in Mechanical Engineering from the University of California, San Diego. Previously, he worked for 12 years at Intuitive Surgical, on the da Vinci Single Port robot product development team. Geoff has been an engineering consultant for dozens of companies in the medical device field. Geoff holds sixteen patents for his designs.
-1.jpeg)
David Mackenzie, Vice President, Product Development
David is a product development professional with over 30 years experience. He started his career at Ethicon designing endoscopic surgical instruments. When Ethicon quadrupled in size in just 2 years, David architected and was the primary developer of one of the medical device industry’s first paperless drawing release systems. For the last 20 years, David has led dozens of product development efforts for Prodigy resulting in successful product launches and several patents. David has also led the latest technology infusion into the Prodigy arsenal: the Industrial Internet of Things (IIoT).
-1.jpeg)
Robyn Miller, VP Sales & Marketing
Robyn Miller is the Vice President of Sales and Marketing at Prodigy and brings over 20 years of experience in sales and sales leadership across the industrial, manufacturing, medical, and utilities sectors primarily relating to innovation and technological advancements. Robyn is passionate about leading with a customer-first approach and has a deep commitment to customer retention and driving growth. Her leadership continues to enhance brand visibility and establish the company as a trusted partner in engineering excellence.
-1.jpeg)
Paul Homan, Business Development Engineer
Paul is a product development professional with several years of experience across various industries. He holds a degree in Mechanical Engineering and is now an Executive MBA candidate, both through The Ohio State University.. Paul started his career at Prodigy working on front-end design and development in the medical device, industrial and consumer products industries before transitioning into his current role. As a Business Development Engineer, Paul works with existing and prospective clients to help them realize their product development goals. Paul's engineering perspective allows him to advocate for the client's needs and offer valuable insights as projects are scoped out.
"Prodigy is more than a vendor. They are a partner who celebrates success, take accountability, and provides avenues to push our projects forward."
Bethany Lawson
Johnson & Johnson MedTech
Resources


.png?width=1800&height=1012&name=Risk%20Management%20in%20Product%20Development%20Projects%20%20A%20Practical%20Guide%20(6).png)
7 Fatal Mistakes in Medical Device Development
Blog post on the 7 Fatal Mistakes in Medical Device Development
Download
Case Study: Gastric Medical Devices
Download
Case Study: Laser Surgical System
Download
Case Study: Midmark Ritter LED Exam Light
Download
On Demand Webinar
From Concept to Protection: Collaborating for Successful Product Innovation
Download
In-House or Outsourced Product Development ROI Guide
DownloadContract Manufacturer vs Prodigy
DownloadFind out how the world's largest medical device companies use Prodigy for their product development needs. Schedule a free consultation.
We welcome the chance to learn more about your company and your product needs.
